The vapour pressure of pure liquids A and B at 400 K are 450 and 700 mm Hg respectively. Find out the composition of liquid mixture if total pressure at this temperature is 600 mm Hg.
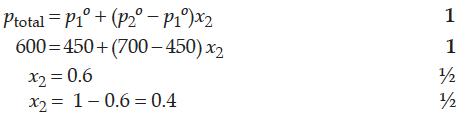
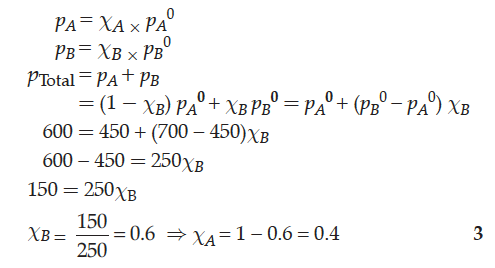
Why a mixture of Carbon disulphide and acetone shows positive deviation from Raoult’s law? What type of azeotrope is formed by this mixture?
The molal elevation constant depends upon
(a) nature of solute.
(b) nature of the solvent.
(c) vapour pressure of the solution.
(d) enthalpy change.
State Henry’s law. What is the effect of temperature on the solubility of a gas in a liquid ?
Define azeotropes. What type of azeotrope is formed by positive deviation from Raoult’s law ?
Give an example.
Explain the solubility rule “like dissolves like” in terms of intermolecular forces that exist in solutions.
What type of intermolecular attractive interaction exists in the pair of methanol and acetone ?
What are colligative properties? Write the colligative property which is used to find the molecular mass of macromolecules.
Consider the reaction: Cr₂O₇²⁻ + 14H⁺ + 6e⁻ -> 2Cr³⁺ + 7H₂O. What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr₂O₇²⁻?
Write the preparation of following :
(i) KMnO₄ from K₂MnO₄
(ii) Na₂CrO₄ from FeCr₂O₄
(iii) Cr₂O₇²⁻ from CrO₄²⁻
(i) Give reasons for the following :
(a) Compounds of transition elements are generally coloured.
(b) MnO is basic while Mn₂O₇ is acidic.
(ii) Calculate the magnetic moment of a divalent ion in aqueous medium if its atomic number is 26.
The magnetic moment of few transition metal ions
are given below:
Calculate the molar conductivity and degree of dissociation.
Conductivity of 2.5 × 10⁻⁴M methanoic acid is 5.25 × 10⁻⁵ Scm⁻¹.
Given : = 50.5Scm² mol⁻¹
The vapour pressure of pure liquids A and B at 400 K are 450 and 700 mm Hg respectively. Find out the composition of liquid mixture if total pressure at this temperature is 600 mm Hg.
(i) Account for the following :
(a) Cu⁺ is unstable in an aqueous solution.
(b) Transition metals form complex compounds.
(ii) Complete the following equation :
CrO₂₇²⁻ + 8H⁺ + 3NO₂⁻ →
