Write the preparation of following :
(i) KMnO₄ from K₂MnO₄
(ii) Na₂CrO₄ from FeCr₂O₄
(iii) Cr₂O₇²⁻ from CrO₄²⁻
(i) KMnO₄ from K₂MnO₄
(ii) Na₂CrO₄ from FeCr₂O₄
(iii) Cr₂O₇²⁻ from CrO₄²⁻
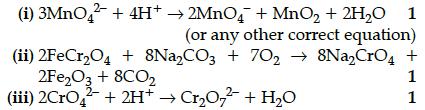
Complete and balance the following chemical equations:
(a) Fe²⁺ + MnO₄⁻ + H⁺ →
(b) MnO₄⁻ + H₂O + I⁻ →
Assign the reason for the following :
Copper (I) ion is not known in aqueous solution.
Complete the following chemical equations :
(i) MnO₄⁻(aq) + S₂O₃²⁻(aq) + H₂O(l) →
(ii) Cr₂O₇²⁻(aq) + Fe²⁺(aq) + H⁺(aq) →
Write the formula of an oxo-anion of Manganese (Mn) in which it shows the oxidation state equal to its group number.
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number.
Complete the following reactions—
(i) Cr₂O₇²⁻ + 6Fe²⁺ + 14H⁺ →
(ii) 2CrO₄²⁻ + 2H⁺ →
(iii) 2MnO₄⁻ + 5C₂O₄²⁻ + 16H⁺ →
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with (i) an iodide (ii) H₂S.
Write the preparation of following :
(i) KMnO₄ from K₂MnO₄
(ii) Na₂CrO₄ from FeCr₂O₄
(iii) Cr₂O₇²⁻ from CrO₄²⁻
Calculate emf of the following cell
Cd/\(Cd^{2+}\) (.10 M)//\(H_+\) (.20 M)/\(H_2\) (0.5 atm)/Pt
[Given E° for \(Cd^{2+}\) /Cd = -0.403V]
Consider the standard electrode potential values (M²⁺/M) of the elements of the first transition series.
When a certain conductance cell was filled with 0.1 M KCl, it has a resistance of 85 ohm at 25°C. When the same cell was filled with an aqueous solution of 0.052 M unknown electrolyte, the resistance was 96 ohms. Calculate the molar conductance of the electrolyte at this concentration.
[Specific conductance of 0.1 M KCl = 1.29 × 10⁻² ohm⁻¹ cm⁻¹]
Give reasons :
(i) Mn shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4.
(ii) Transition metals show variable oxidation states.
(iii) Actinoids show irregularities in their electronic configurations.
Complete the following reactions—
(i) Cr₂O₇²⁻ + 6Fe²⁺ + 14H⁺ →
(ii) 2CrO₄²⁻ + 2H⁺ →
(iii) 2MnO₄⁻ + 5C₂O₄²⁻ + 16H⁺ →
The electrical resistance of a column of 0.05 M KOH solution of diameter 1 cm and length 45.5 cm is 4.55 × 10³ ohm. Calculate its molar conductivity.
