(a) 30 g of urea (M = 60 g mol-1) is dissolved in 846 g of water. Calculate the vapour pressure of water for this solution if vapour pressure of pure water at 298 K is 23.8 mm Hg.
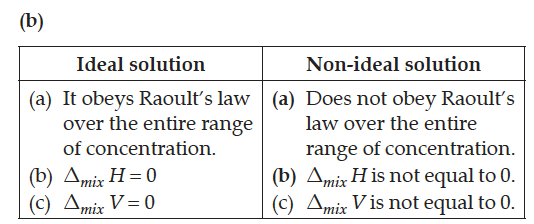
(b) Write two differences between ideal solutions and non-ideal solutions.
(b) Write two differences between ideal solutions and non-ideal solutions.
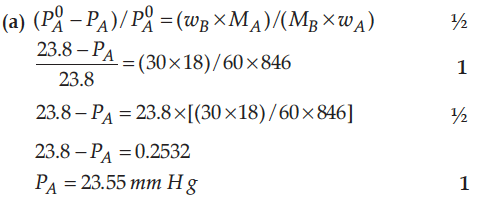
Explain why on addition of 1 mol of NaCl to 1 litre of water, the boiling point of water increases, while addition of 1 mol of methyl alcohol to one litre of water decreases its boiling point.
Which of the following aqueous solutions containing 10 g of solute in each case, has highest m.p.?
(a) NaCl solution
(b) KCl solution
(c) sugar solution
(d) glucose solution
The molal elevation constant depends upon
(a) nature of solute.
(b) nature of the solvent.
(c) vapour pressure of the solution.
(d) enthalpy change.
The osmotic pressure of a solution is directly proportional to
(a) the molecular concentration of the solute.
(b) the absolute temperature at a given concentration.
(c) the lowering of vapour pressure.
(d) all the above.
When mercuric iodide is added to the aqueous solution of potassium iodide, the:
(a) Freezing point is raised
(b) Freezing point does not change
(c) Freezing point is lowered
(d) Boiling point does not change
State Raoult’s law for a solution containing nonvolatile solute. What type of deviation from Raoult’s law is shown by a solution of chloroform and acetone and why?
State Raoult’s law for the solution containing volatile components. What is the similarity between Raoult’s law and Henry’s law ?
(a) 30 g of urea (M = 60 g mol-1) is dissolved in 846 g of water. Calculate the vapour pressure of water for this solution if vapour pressure of pure water at 298 K is 23.8 mm Hg.
(b) Write two differences between ideal solutions and non-ideal solutions.
