Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes:
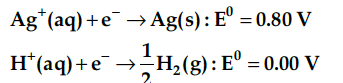
On the basis of their standard electrode potential values, which reaction is feasible at cathode and why?
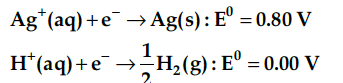
On the basis of their standard electrode potential values, which reaction is feasible at cathode and why?
As reaction with higher value of standard electrode potential occurs at cathode, Ag gets reduced. So, the reaction occurring at cathode is Ag⁺ (aq)+e⁻ (s) -> Ag(s)
The conductivity of metals decreases while that of electrolytes increases with increase in temperature. Why?
State Kohlrausch law of independent migration of ions. Why does the conductivity of a solution decrease with dilution ?
The electrical resistance of a column of 0.05 M KOH solution of diameter 1 cm and length 45.5 cm is 4.55 × 10³ ohm. Calculate its molar conductivity.
The conductivity of an aqueous solution of NaCl in a cell is 92 \(Ω^{−1}\) \(cm^{-1}\) the resistance offered by this cell is 247.8 Ω . Calculate the cell constant.
Calculate pH of following half-cell. Pt, \(H_2\) / \(H_2\)\(SO_4\) , if its electrode potential is 0.03V.
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ___________.
a. Cell potential.
b. Electromotive Force.
c. Potential difference.
d. Cell voltage.
How many electrons flow when a current of 5 amps is passed through a solution for 193 sec ? Given F = 96500 C. \(N_0\) = 6.002 × \(10^{23}\) \(mol^{-1}\).
How many electrons flow when a current of 5 amps is passed through a solution for 193 sec ? Given F = 96500 C. \(N_0\) = 6.002 × \(10^{23}\) \(mol^{-1}\).
There are two possible reactions for cathode in the electrolysis of aqueous
\(ZnCl_2\) :\(Zn^{2+}\) (aq) + 2\(e^-\) → Zn(s) \(E^0\) = -0.76 V
2\(H_2\)O (l) + 2\(e^-\) → H2 (g) + 2\(OH^-\) (aq) \(E^0\) = - 0.83 V
Which one will take place? Why?
Suggest reasons for the following features of transition metal chemistry :
(i) The transition metals and their compounds are usually paramagnetic.
(ii) The transition metals exhibit variable oxidation states.
Calculate the molality of ethanol solution in which the mole fraction of water is 0.88.
The conductivity of a 0.01 M solution of acetic acid at 298 K is 1.65 x 10⁻⁴ S cm⁻¹. Calculate molar conductivity () of the solution.
(i) Write the colligative property which is used to find the molecular mass of macromolecules.
(ii) In non-ideal solution, what type of deviation shows the formation of minimum boiling azeotropes?
