Looking at the setup of an electrochemical cell, what happens when \(E_{ext}\) > 1.1 V
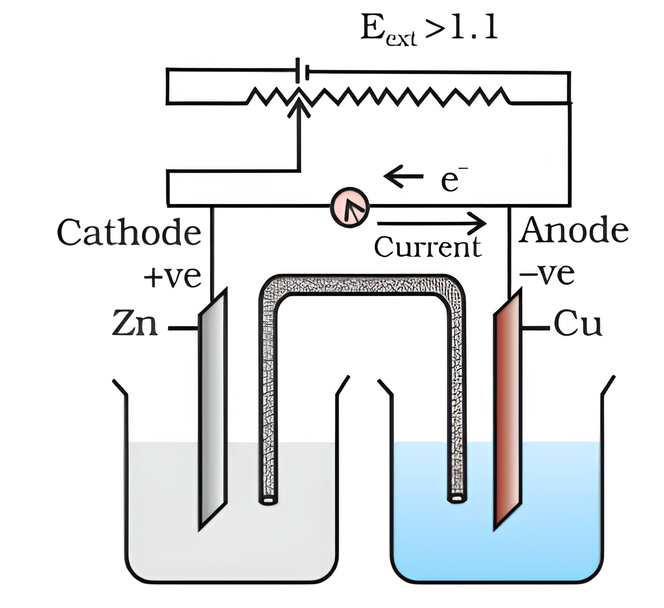
a. Zn dissolves at anode & copper deposits at cathode
b. Current travels from Cu to Zn
c. Zinc deposits at anode and copper dissolves at cathode.
d. No current is obtained

a. Zn dissolves at anode & copper deposits at cathode
b. Current travels from Cu to Zn
c. Zinc deposits at anode and copper dissolves at cathode.
d. No current is obtained
c. Zinc deposits at anode and copper dissolves at cathode.
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ___________.
a. Cell potential.
b. Electromotive Force.
c. Potential difference.
d. Cell voltage.
The electrical resistance of a column of 0.05 M KOH solution of diameter 1 cm and length 45.5 cm is 4.55 × 10³ ohm. Calculate its molar conductivity.
Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes:
The conductivity of an aqueous solution of NaCl in a cell is 92 \(Ω^{−1}\) \(cm^{-1}\) the resistance offered by this cell is 247.8 Ω . Calculate the cell constant.
State Kohlrausch law of independent migration of ions. Why does the conductivity of a solution decrease with dilution ?
How is electrical conductance of a conductor related with length and area of cross-section of the conductor?
a. G = \(l. a.k^{-1}\)
b. G = \(k. l.a^{-1}\)
c. G = \(k.a. l^{-1}\)
d. G = \(k. l.a^{-2}\)
The conductivity of metals decreases while that of electrolytes increases with increase in temperature. Why?
Which of the following is a network solid?
(A) (solid)
(B)
(C) Diamond
(D) (ice)
Which stoichiometric defect does not change the density of the crystal?
(A) Frenkel defect
(B) Schottky defect
(C) Interstitial defect
(D) F-centres
Benzene reacts with \(CH_3\)Cl in the presence of anhydrous \(AlCl_3\) to form:
(a) chlorobenzene
(b) benzylchloride
(c) xylene
(d) toluene
Aryl halides can not be prepared by the reaction of arylalcohols with \(PCl_3\), \(PCl_5\) or \(SOCl_2\) because :
(a) phenols are highly stable compounds.
(b) carbon-oxygen bond in phenols has a partial doublebond character.
(c) carbon-oxygen bond is highly polar
(d) all of these
Chlorobenzene is formed by reaction of chlorine with benzene in the presence of \(AlCl_3\). Which of the following species attacks the benzene ring in this reaction?
(a) \(Cl^+\)
(b) \(Cl^-\)
(c) \(AlCl_3\)
(d) \({[\\(AlCl_4\\)]}^-\)
When Xe reacts with Fluorine in 1:5 ratio at 873 K it forms
a) \(XeF_2\)
b) \(XeF_4\)
c) \(XeF_6\)
d) \(XeOF_4\)
The correct order of the packing efficiency in different types of unit cells is ________.
(A) fcc < bcc < simple cubic
(B) fcc > bcc > simple cubic
(C) fcc < bcc > simple cubic
(D) bcc < fcc = simple cubic
