Calculate the degree of dissociation (α) of acetic acid if its molar conductivity () is 39.05 Scm² mol⁻¹. Given (H⁺) = 349.6 Scm² mol⁻¹ and (CH₃COO⁻) = 40.9 Scm² mol⁻¹..
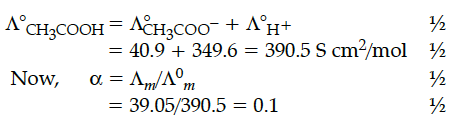
The molar conductivity of 0.025 mol L⁻¹ methanoic acid is 46.1 S cm² mol⁻¹. Calculate its degree of dissociation and dissociation constant. Given λ°(H⁺) = 349.6 S cm² mol⁻¹ and λ°(HCOO⁻) = 54.6 S cm² mol⁻¹.
The conductivity of a 0.01 M solution of acetic acid at 298 K is 1.65 x 10⁻⁴ S cm⁻¹. Calculate molar conductivity () of the solution.
Calculate the molar conductivity and degree of dissociation.
Conductivity of 2.5 × 10⁻⁴M methanoic acid is 5.25 × 10⁻⁵ Scm⁻¹.
Given : = 50.5Scm² mol⁻¹
(i) State the law which helps to determine the limiting molar conductivity of weak electrolyte.
(ii) Calculate limiting molar conductivity of CaSO₄ (limiting molar conductivity of calcium and sulphate ions are 119.0 and 160.0 Scm² mol⁻¹ respectively)
Calculate emf of the following cell
Cd/\(Cd^{2+}\) (.10 M)//\(H_+\) (.20 M)/\(H_2\) (0.5 atm)/Pt
[Given E° for \(Cd^{2+}\) /Cd = -0.403V]
The following curve is obtained when molar conductivity is plotted against the square root of concentration, c½ for two electrolytes A and B :
Calculate the molality of ethanol solution in which the mole fraction of water is 0.88.
What is meant by ‘disproportionation’ ? Give an example of a disproportionation reaction in aqueous solution.
A 1.00 molar aqueous solution of trichloroacetic acid (CCl₃COOH) is heated to its boiling point. The solution has the boiling point of 100.18 °C. Determine the van’t Hoff factor for trichloroacetic acid. ( for water = 0.512 K kg mol⁻¹).
Why a mixture of Carbon disulphide and acetone shows positive deviation from Raoult’s law? What type of azeotrope is formed by this mixture?
Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes:
