The following curve is obtained when molar conductivity is plotted against the square root of concentration, c½ for two electrolytes A and B :
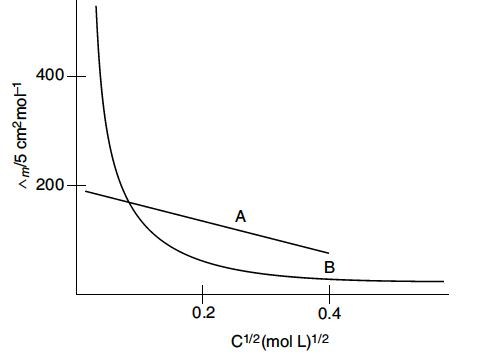
(i) How do you account for the increase in the molar conductivity of the electrolyte A on dilution ?
(ii) As seen from the graph, the value of limiting molar conductivity for electrolyte B cannot be obtained graphically. How can this value be obtained ?

(i) How do you account for the increase in the molar conductivity of the electrolyte A on dilution ?
(ii) As seen from the graph, the value of limiting molar conductivity for electrolyte B cannot be obtained graphically. How can this value be obtained ?
(i) As seen from the graph, electrolyte A is a strong electrolyte which is completely ionised in solution. With dilution, the ions are far apart from each other and hence the molar conductivity increases.
(ii) To determine the value of limiting molar conductivity for electrolyte B, indirect method based upon Kohlrausch law of independent migration of ions is used.
(ii) To determine the value of limiting molar conductivity for electrolyte B, indirect method based upon Kohlrausch law of independent migration of ions is used.
(i) State the law which helps to determine the limiting molar conductivity of weak electrolyte.
(ii) Calculate limiting molar conductivity of CaSO₄ (limiting molar conductivity of calcium and sulphate ions are 119.0 and 160.0 Scm² mol⁻¹ respectively)
Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes:
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called ___________.
a. Cell potential.
b. Electromotive Force.
c. Potential difference.
d. Cell voltage.
Looking at the setup of an electrochemical cell, what happens when \(E_{ext}\) > 1.1 V
Out of the following pairs, predict with reason which pair will allow greater conduction of electricity:
(i) Silver wire at 30°C or silver wire at 60°C.
(ii) 0.1 M \(CH_3\)COOH solution or 1 M \(CH_3\)COOH solution.
(iii) KCl solution at 20°C or KCl solution at 50°C.
The conductivity of an aqueous solution of NaCl in a cell is 92 \(Ω^{−1}\) \(cm^{-1}\) the resistance offered by this cell is 247.8 Ω . Calculate the cell constant.
How is electrical conductance of a conductor related with length and area of cross-section of the conductor?
a. G = \(l. a.k^{-1}\)
b. G = \(k. l.a^{-1}\)
c. G = \(k.a. l^{-1}\)
d. G = \(k. l.a^{-2}\)
State Henry’s law. What is the effect of temperature on the solubility of a gas in a liquid ?
Why a mixture of Carbon disulphide and acetone shows positive deviation from Raoult’s law? What type of azeotrope is formed by this mixture?
The conductivity of an aqueous solution of NaCl in a cell is 92 \(Ω^{−1}\) \(cm^{-1}\) the resistance offered by this cell is 247.8 Ω . Calculate the cell constant.
Define the following terms :
(i) Mole fraction
(ii) Isotonic solutions
(iii) Van’t Hoff factor
(iv) Ideal solution
Complete and balance the following chemical equations:
(a) Fe²⁺ + MnO₄⁻ + H⁺ →
(b) MnO₄⁻ + H₂O + I⁻ →
Define the following terms :
(i) Molar conductivity (),
(ii) Secondary batteries.
Calculate the molality of ethanol solution in which the mole fraction of water is 0.88.
