Which of the following compounds will be most easily attacked by an electrophile?
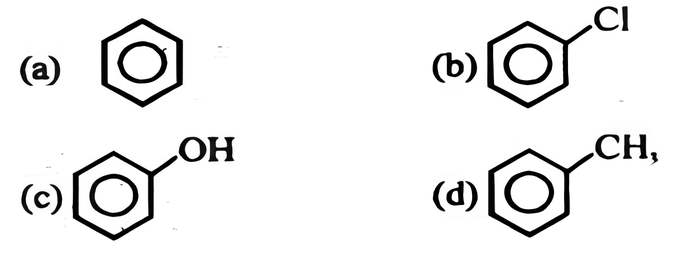
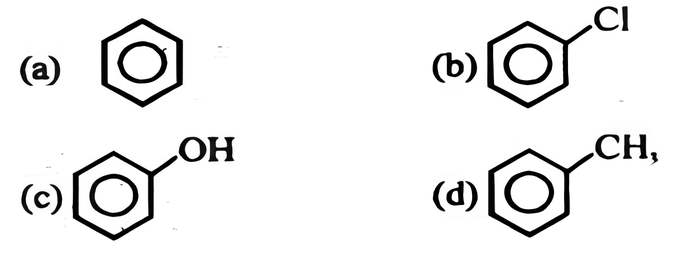
(c) phenol.
Order of esterification of alcohols are
(a) 3° > 1° > 2°.
(b) 2°> 3° > 1°.
(c) 1 ° > 2° > 3°.
(d) None of these.
Which of the following alcohols will give the most stable carbocation during dehydration?
(a) 2-methyl-1-propanol.
(b) 2-methyl-2-propanol.
(c) 1-Butanol.
(d) 2-Butanol.
Which of the following alcohols gives 2-butenc on dehydration by conc.\(H_2\)\(SO_4\)?
(a) 2-methyl propene-2-ol.
(b) 2-methyl 1 -propanol.
(c) Butane-2-ol.
(d) Butane 1-ol.
Propanone on reaction with alkyl magnesium bromide followed by hydrolysis will produce
(a) primary alcohol
(b) secondary alcohol
(c) tertiary alcohol
(d) carboxylic acid
1-Phenylethanol can be prepared by the reaction of benzaldehyde with
(a) methyl bromide.
(b) ethyl iodide and magnesium.
(c) methyl iodide and magnesium.
(d) methyl bromide and aluminium bromide.
What happens when tertiary butyl alcohol is passed over heated copper at 300°C?
(a) Secondary butyl alcohol is formed.
(b) 2-methylpropene is formed.
(c) 1-butene is formed.
(d) Butanol is formed.
An antifreeze solution is prepared from 222.6 g of ethylene glycol \(C_2\)\(H_4\)\((OH)_2\) and 200 g of water. Calculate the molality of the solution. If the density of this solution be 1.072 \(gmL^{-1}\), what will be the molarity of the solution?
(a) 7.20 M
(b) 12.03 M
(c) 9.11 M
(d) 6 M
There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to
a) Increase in number of shells
b) increase in valence electrons
c) increase in ionisation enthalpy
d) the presence of completely filled d and/or f orbitals
The idea that promoted to Bartlett to prepare first ever compound of Noble gases was
a) Low bond dissociation enthalpy of F-F bond in \(F_2\).
b) High bond enthalpy of Xe-F bond.
c) Ionization enthalpies of \(O_2\) and Xe are almost same.
d) None of the above.
The reason for double helical structure of DNA is operation of-
(a) electrostatic attractions
(b) dipole-dipole interaction
(c) van der Waal’s forces
(d) hydrogen bonding
Which of the following statements is not true about amorphous solids?
(A) On heating they may become crystalline at certain temperature.
(B) They may become crystalline on keeping for long time.
(C) Amorphous solids can be moulded by heating.
(D) They are anisotropic in nature.
The decreasing order of boiling point of the following alcohols is
(a) 3-methylbuan-2-ol > 2-methylbutan-2-ol > pentan-1-ol.
(b) Pentan-1-ol > 3-methylbutan-2-ol > 2-methylbutan-2-ol.
(c) 2-methylbutan-2-ol > 3-methylbutan-2-ol > pentan-1-ol.
(d) 2-methylbutan-2-ol > pental-1-ol > 3-methylbutan-2-ol.
A compound is formed by two elements M and N. The element N forms ccp lattice and atoms of M occupy two atoms an Mercury 1/3rd of tetrahedral voids. What is the formula of the compound?
(A) \(MN_2\)
(B) \(M_2\)\(N_3\)
(C) \(M_3\)\(N_2\)
(D) \(M_2\)\(N_2\)
