Which of the following alcohols reacts most readily with Lucas reagent?
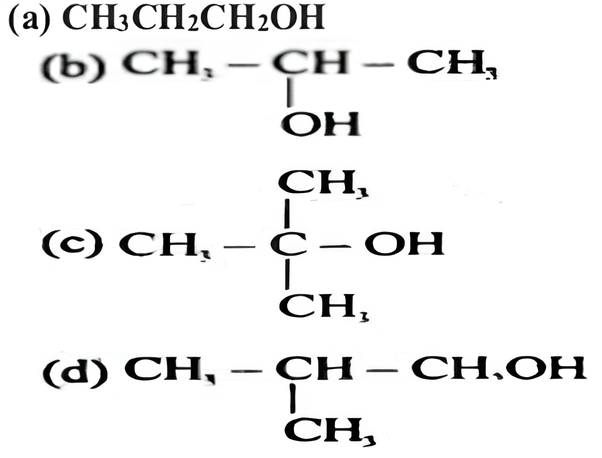

(c) tert. Butyl alcohol 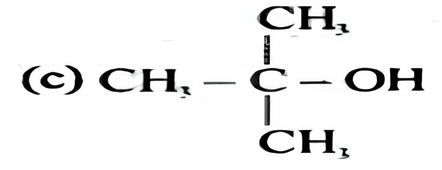
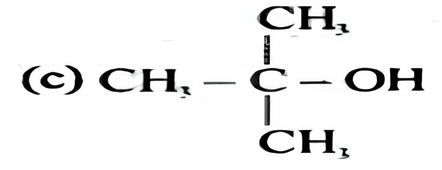
Order of esterification of alcohols are
(a) 3° > 1° > 2°.
(b) 2°> 3° > 1°.
(c) 1 ° > 2° > 3°.
(d) None of these.
Which of the following alcohols will give the most stable carbocation during dehydration?
(a) 2-methyl-1-propanol.
(b) 2-methyl-2-propanol.
(c) 1-Butanol.
(d) 2-Butanol.
Which of the following alcohols gives 2-butenc on dehydration by conc.\(H_2\)\(SO_4\)?
(a) 2-methyl propene-2-ol.
(b) 2-methyl 1 -propanol.
(c) Butane-2-ol.
(d) Butane 1-ol.
Propanone on reaction with alkyl magnesium bromide followed by hydrolysis will produce
(a) primary alcohol
(b) secondary alcohol
(c) tertiary alcohol
(d) carboxylic acid
What happens when tertiary butyl alcohol is passed over heated copper at 300°C?
(a) Secondary butyl alcohol is formed.
(b) 2-methylpropene is formed.
(c) 1-butene is formed.
(d) Butanol is formed.
1-Phenylethanol can be prepared by the reaction of benzaldehyde with
(a) methyl bromide.
(b) ethyl iodide and magnesium.
(c) methyl iodide and magnesium.
(d) methyl bromide and aluminium bromide.
There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to
a) Increase in number of shells
b) increase in valence electrons
c) increase in ionisation enthalpy
d) the presence of completely filled d and/or f orbitals
An alcohol X when treated with hot conc. \(H_2\)\(SO_4\) gave an alkene Y with formula \(C_4\)\(H_8\). This alkene on ozonolysis gives single product with molecular formula \(C_2\)\(H_4\)O. The alcohol is
(a) butan-1-ol.
(b) butan-2-ol.
(c) 2-methylpropan-1-ol.
(d) 2,2-dimethylbutynal-1-oI.
Which of the following statements is not true about the hexagonal close packing?
(A) The coordination number is 12.
(B) It has 74% packing efficiency.
(C) Tetrahedral voids of the second layer are covered by the spheres of the third layer.
(D) In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
Which of the following conditions favours the existence of a substance in the solid state?
(A) High temperature
(B) Low temperature
(C) High thermal energy
(D) Weak cohesive forces
Chlorobenzene is formed by reaction of chlorine with benzene in the presence of \(AlCl_3\). Which of the following species attacks the benzene ring in this reaction?
(a) \(Cl^+\)
(b) \(Cl^-\)
(c) \(AlCl_3\)
(d) \({[\\(AlCl_4\\)]}^-\)
Identify the correct order of acidic strength for H-X
a) HCl > HBr > HI.
b) HCl > HI > HBr.
c) HI > HCl > HBr.
d) HI > HBr > HCl.
Hypochlorous acid and perchloric acid are, respectively:
a) HOCl and \(HClO_4\).
b) HOCl and \(HClO_3\).
c) \(HClO_2\) and \(HClO_3\).
d) \(HClO_2\) and \(HClO_4\).
