The partial pressure of ethane over a saturated solution containing 6.56 × 10⁻² g of ethane is 1 bar. If the solution were to contain 5.0 × 10⁻² g of ethane, then what will be the partial pressure of the gas ?
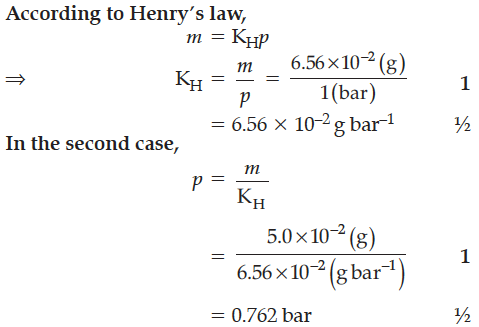
State Raoult’s law for the solution containing volatile components. What is the similarity between Raoult’s law and Henry’s law ?
The vapour pressure of pure liquids A and B at 400 K are 450 and 700 mm Hg respectively. Find out the composition of liquid mixture if total pressure at this temperature is 600 mm Hg.
State Raoult’s law for a solution containing nonvolatile solute. What type of deviation from Raoult’s law is shown by a solution of chloroform and acetone and why?
Which of the following aqueous solutions containing 10 g of solute in each case, has highest m.p.?
(a) NaCl solution
(b) KCl solution
(c) sugar solution
(d) glucose solution
The molal elevation constant depends upon
(a) nature of solute.
(b) nature of the solvent.
(c) vapour pressure of the solution.
(d) enthalpy change.
The osmotic pressure of a solution is directly proportional to
(a) the molecular concentration of the solute.
(b) the absolute temperature at a given concentration.
(c) the lowering of vapour pressure.
(d) all the above.
Why a mixture of Carbon disulphide and acetone shows positive deviation from Raoult’s law? What type of azeotrope is formed by this mixture?
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with (i) an iodide (ii) H₂S.
What are the transition elements ? Write two characteristics of the transition elements.
Explain why on addition of 1 mol of NaCl to 1 litre of water, the boiling point of water increases, while addition of 1 mol of methyl alcohol to one litre of water decreases its boiling point.
Define the following terms :
(i) Mole fraction (x)
(ii) Molality of a solution (m)
In the following ions:
Mn³⁺, V³⁺, Cr³⁺, Ti⁴⁺
(Atomic no: Mn = 25, V = 23, Cr = 24, Ti = 22)
(a) Which ion is most stable in an aqueous solution?
(b) Which ion is the strongest oxidizing agent?
(c) Which ion is colourless?
(d) Which ion has the highest number of unpaired electrons?
State Raoult’s law for the solution containing volatile components. What is the similarity between Raoult’s law and Henry’s law ?
Define the following terms:
(i) Abnormal molar mass
(ii) van’t Hoff factor
