Define the following terms :
(i) Mole fraction (x)
(ii) Molality of a solution (m)
(i) Mole fraction (x)
(ii) Molality of a solution (m)
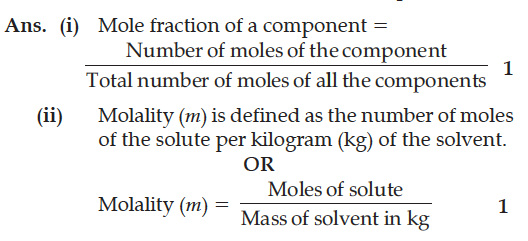
Define the following terms :
(i) Mole fraction
(ii) Isotonic solutions
(iii) Van’t Hoff factor
(iv) Ideal solution
Calculate pH of following half-cell. Pt, \(H_2\) / \(H_2\)\(SO_4\) , if its electrode potential is 0.03V.
State Henry’s law. What is the effect of temperature on the solubility of a gas in a liquid ?
What is meant by ‘disproportionation’ ? Give an example of a disproportionation reaction in aqueous solution.
State Kohlrausch law of independent migration of ions. Why does the conductivity of a solution decrease with dilution ?
What are the transition elements ? Write two characteristics of the transition elements.
Define the following terms:
(i) Abnormal molar mass
(ii) van’t Hoff factor
State Raoult’s law. How is it formulated for solutions of non-volatile solutes ?
