Define the following terms :
(i) Isotonic solutions
(ii) Van’t Hoff factor
(i) Isotonic solutions
(ii) Van’t Hoff factor
(i) The solutions having same osmotic pressure at a given temperature are called isotonic solutions.
(ii) Van’t Hoff factor is expressed as :
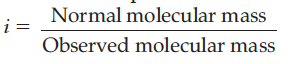
(ii) Van’t Hoff factor is expressed as :
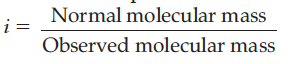
Define the following terms :
(i) Mole fraction
(ii) Isotonic solutions
(iii) Van’t Hoff factor
(iv) Ideal solution
Define the following terms :
(i) Isotonic solutions
(ii) Van’t Hoff factor
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number.
How would you account for the following ? Many of the transition elements are known to form interstitial compounds.
