What is meant by rate of a reaction ? Differentiate between average rate and instantaneous rate of a reaction.
Rate of a reaction is defined as the change in concentration of reactant or product in a chemical reaction at particular time interval.
Rate of reaction
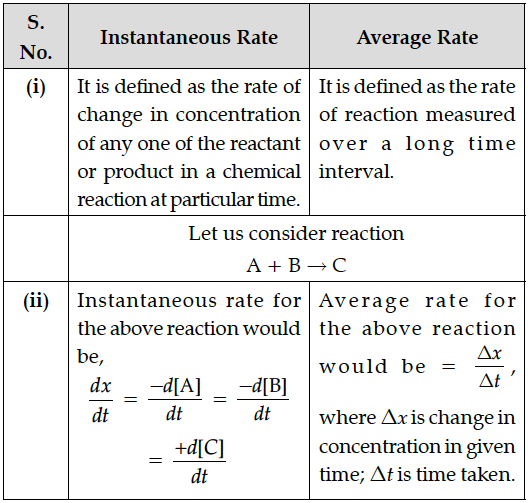
Rate of reaction

Define the following terms :
(i) Mole fraction (x)
(ii) Molality of a solution (m)
Explain the following observations:
(i) Copper atom has completely filled d orbitals (3d¹⁰) in its ground state, yet it is regarded as a transition element.
(ii) Cr²⁺ is a stronger reducing agent than Fe²⁺ in aqueous solution.
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with (i) an iodide (ii) H₂S.
The conductivity of a 0.01 M solution of acetic acid at 298 K is 1.65 x 10⁻⁴ S cm⁻¹. Calculate molar conductivity () of the solution.
State Raoult’s law for a solution containing nonvolatile solute. What type of deviation from Raoult’s law is shown by a solution of chloroform and acetone and why?
