The solubility of gases increases with increase of pressure. William Henry made a systematic investigation of the solubility of a gas in a liquid. According to Henry’s law “the mass of a gas dissolved per unit volume of the solvent at constant temperature is directly proportional to the pressure of the gas in equilibrium with the solution”. Dalton during the same period also concluded independently that the solubility of a gas in a liquid solution depends upon the partial pressure of the gas. If we use the mole fraction of gas in the solution as a measure of its solubility, then Henry’s law can be modified as “the partial pressure of the gas in the vapour phase is directly proportional to the mole fraction of the gas in the solution”.
Answer the following MCQs by choosing the most appropriate options:
(i) Henry’s law constant for the solubility of methane in benzene at 298 K is 4.27 × \(10^5\) mm Hg. The solubility of methane in benzene at 298 K under 760 mm Hg is
(a) 4.27 × \(10^{-5}\)
(b) 1.78 × \(10^{-3}\)
(c) 4.27 × \(10^{-3}\)
(d) 1.78 × \(10^{-3}\)
(ii) The partial pressure of ethane over a saturated solution containing 6.56 × \(10^{-2}\) g of ethane is 1 bar. If the solution contains 5.00 × \(10^{-2}\) g of ethane then what will be the partial pressure (in bar) of the gas?
(a) 0.762
(b) 1.312
(c) 3.81
(d) 5.0
(iii) KH (K bar) values for Ar(g), \(CO_2\)(g), HCHO(g) and \(CH_4\)(g) are 40.39, 1.67, 1.83 × \(10^{-5}\) and 0.413 respectively. Arrange these gases in the order of their increasing solubility.
(a) HCHO < \(CH_4\) < \(CO_2\) < Ar
(b) HCHO < \(CO_2\) < \(CH_4\) < Ar
(c) Ar < \(CO_2\) < \(CH_4\) < HCHO
(d) Ar < \(CH_4\) < \(CO_2\) < HCHO
(iv) When a gas is bubbled through water at 298 K, a very dilute solution of the gas is obtained. Henry’s law constant for the gas at 298 K is 150 K bar. If the gas exerts a partial pressure of 2 bar, the number of millimoles of the gas dissolved in 1 L of water is
(a) 0.55
(b) 0.87
(c) 0.37
(d) 0.66
Answer the following MCQs by choosing the most appropriate options:
(i) Henry’s law constant for the solubility of methane in benzene at 298 K is 4.27 × \(10^5\) mm Hg. The solubility of methane in benzene at 298 K under 760 mm Hg is
(a) 4.27 × \(10^{-5}\)
(b) 1.78 × \(10^{-3}\)
(c) 4.27 × \(10^{-3}\)
(d) 1.78 × \(10^{-3}\)
(ii) The partial pressure of ethane over a saturated solution containing 6.56 × \(10^{-2}\) g of ethane is 1 bar. If the solution contains 5.00 × \(10^{-2}\) g of ethane then what will be the partial pressure (in bar) of the gas?
(a) 0.762
(b) 1.312
(c) 3.81
(d) 5.0
(iii) KH (K bar) values for Ar(g), \(CO_2\)(g), HCHO(g) and \(CH_4\)(g) are 40.39, 1.67, 1.83 × \(10^{-5}\) and 0.413 respectively. Arrange these gases in the order of their increasing solubility.
(a) HCHO < \(CH_4\) < \(CO_2\) < Ar
(b) HCHO < \(CO_2\) < \(CH_4\) < Ar
(c) Ar < \(CO_2\) < \(CH_4\) < HCHO
(d) Ar < \(CH_4\) < \(CO_2\) < HCHO
(iv) When a gas is bubbled through water at 298 K, a very dilute solution of the gas is obtained. Henry’s law constant for the gas at 298 K is 150 K bar. If the gas exerts a partial pressure of 2 bar, the number of millimoles of the gas dissolved in 1 L of water is
(a) 0.55
(b) 0.87
(c) 0.37
(d) 0.66
(i) (b) 1.78 × \(10^{-3}\)
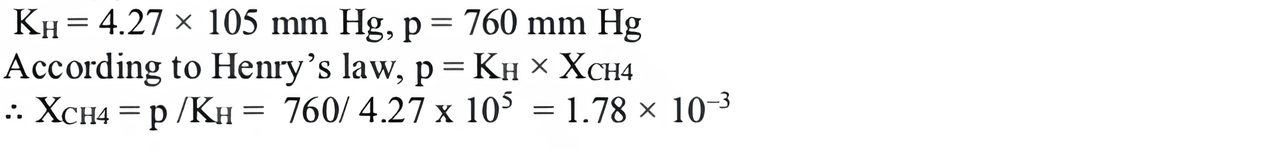
(ii) (a) 0.762
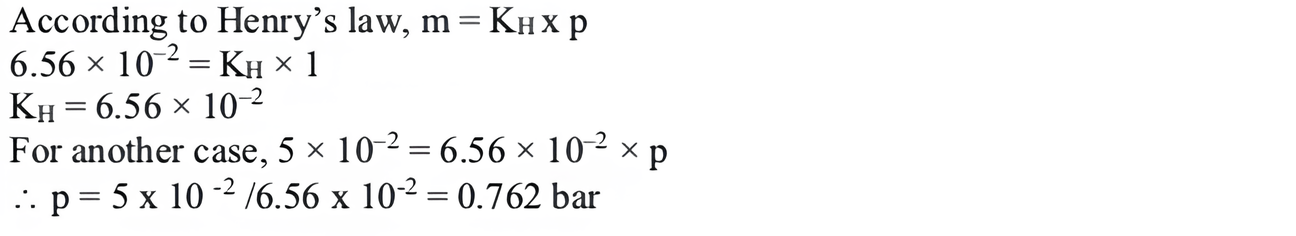
(iii) (c) Ar < \(CO_2\) < \(CH_4\) < HCHO

(iv) (c) 0.37

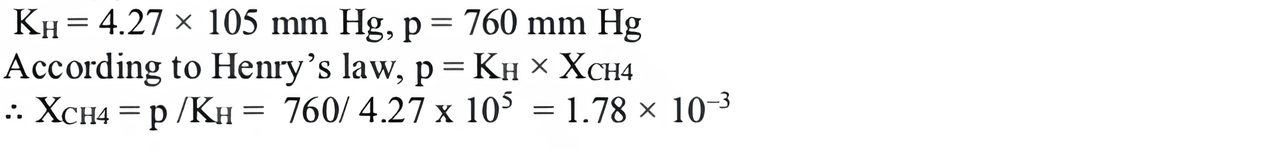
(ii) (a) 0.762
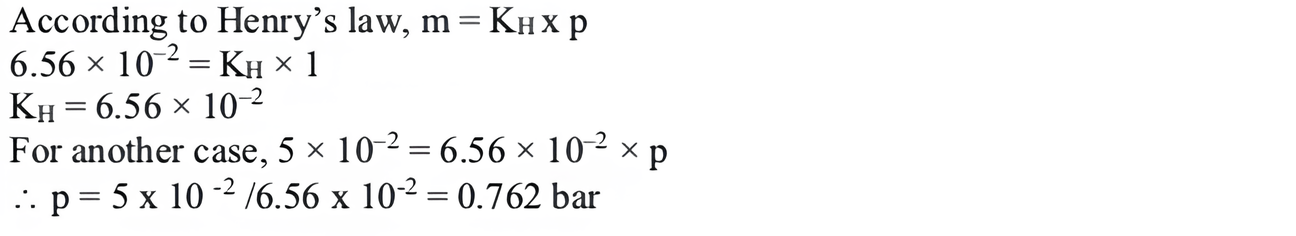
(iii) (c) Ar < \(CO_2\) < \(CH_4\) < HCHO

(iv) (c) 0.37

The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties. For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg.(vapour pressure of water at \(20^\circ\)C is 17.5 mm of Hg).
Answer the following MCQs by choosing the most appropriate options:
(i) Relative lowering of vapour pressure for the given solution is-
(a) 0.00348
(b) 0.061
(c) 0.122
(d) 1.75
(ii) The vapour pressure (mm of Hg) of Solution will be
(a) 17.5
(b) 0.61
(c) 17.439
(d) 0.00348
(iii) Mole fraction of sugar in the solution is
(a) 0.00348
(b) 0.9965
(c) 0.061
(d) 1.75
(iv) If weight of sugar taken is 5 g in 108 g of water then molar mass of sugar will be
(a) 358
(b) 120
(c) 240
(d) 400
(v) The vapour pressure (mm of Hg) of water at 293 K when 25 g of glucose is dissolved in 450 g of water is
(a) 17.2
(b) 17.4
(c) 17.120
(d) 17.02
The properties of dilute or ideal solutions which depend only upon the concentration of the solute in the solution and no other characteristics are known as colligative properties. There are in all four such properties i.e. relative lowering in vapour pressure, osmotic pressure, elevation in boiling point temperature and depression in freezing point temperature. All of them help in calculating the observed molar mass of the solute which is inversely proportional to the colligative property involved. Out of these, osmotic pressure may be regarded as the best for the determination of molecular mass of the solute. According to Van’t Hoff theory of dilute solution, π = CRT, where ‘π’ is the osmotic pressure while ‘C’ is the molar concentration of the solution.
(i) When liquids A and B are mixed, hydrogen bonding occurs. The solutions will show:
a) Positive deviation from Raoult’s law
b) Negative deviation from Raoult’s law
c) No deviation from Raoult’s law
d) Slightly increase in volume
(ii) The azeotropic mixture of water and HCl boils at \(108.5^\circ\)C when the mixture is distilled. It is possible to obtain:
a) Pure HCl
b) Pure water
c) Pure water as well as pure HCl
d) Neither HCl nor water in their pure states.
(iii) On freezing an aqueous solution of sugar, the solid which starts separating out is:
a. Sugar
b. Ice
c. Solution with the same composition
d. Solution with different composition
(iv) The value of osmotic pressure does not depend upon:
a) Concentration of the solution
b) Temperature of the solution
c) Number of the particles of the solute present
d) Structure of the solute particles
(v) Effect of adding a non-volatile solute to a solvent is :
a) to lower the vapour pressure
b) to increase the freezing point
c) to decrease the boiling point
d) to decrease the osmotic pressure
Acetone and carbon disulphide form binary liquid solution showing positive deviation from Raoult’s law. The normal boiling point (Tb) of pure acetone is less than that of pure \(CS_2\). Pick out the
incorrect statement among the following-
(a) Boiling temperature of the mixture is always less than the boiling temperature of acetone
(b) Boiling temperature of Azeotropic mixture is always less than the boiling temperature of acetone
(c) When a small amount of \(CS_2\) (less volatile component) is added to an excess of acetone boiling point
of the resulting mixture increases
(d) A mixture of \(CS_2\) and \(CH_3\)\(COCH_3\) can be completely separated by simple fractional distillation
Consider the figure and mark the correct option.
(a) water will move from side (A) to side (B) if a pressure lower than osmotic pressure is applied on piston (B).
(b) water will move from side (B) to side (A) if a pressure greater than osmotic , pressure is applied on piston (B).
(c) water will move from side (B) to side (A) if a pressure equal to osmotic pressure is applied on piston (B).
(d) water will move from side (A) to side (B) if pressure equal to osmotic pressure is applied on piston (A).
Assertion: 1 M glucose will have a higher boiling point than 2 M glucose.
Reason: Elevation in boiling point is a colligative property which depends upon the number of particles of solute in the solution.
A. If both Assertion and Reason are correct and reason is the correct explanation of Assertion .
B. If both Assertion and Reason are correct and reason is not correct explanation of Assertion
C. If Assertion is correct but Reason is incorrect.
D. If Assertion is incorrect and Reason is correct.
Assertion: An aqueous solution of NaCl freezes below 273 K.
Reason: Vapour pressure of the solution is less than that of the pure solvent.
A. If both Assertion and Reason are correct and reason is the correct explanation of Assertion .
B. If both Assertion and Reason are correct and reason is not correct explanation of Assertion
C. If Assertion is correct but Reason is incorrect.
D. If Assertion is incorrect and Reason is correct.
Assertion: Isotonic solutions do not show any osmosis when placed side by side.
Reason: Isotonic solutions have same solute concentration.
A. If both Assertion and Reason are correct and reason is the correct explanation of Assertion .
B. If both Assertion and Reason are correct and reason is not correct explanation of Assertion
C. If Assertion is correct but Reason is incorrect.
D. If Assertion is incorrect and Reason is correct.
Group 18 elements are called noble gases and not inert gases because compounds of Kr, Xe and Rn have been prepared. Their general electronic configuration is \(ns^2\)\(np^6\) except He(\(1s^2\) ). They have highest ionisation enthalpy and positive electron gain enthalpy due to stable electronic configuration. Helium is found in sun and stars. Noble gases have low boiling points due to weak van der Waals’ forces of attraction. Xenon forms \(XeF_2\), \(XeF_4\), \(XeF_6\), \(XeOF_4\), \(XeO_3\), \(XeO_2\)\(F_2\), their structures can be drawn on bases of VSEPR theory. Helium is mixed with oxygen by deep sea divers to avoid pain. Neon is used in coloured advertising lights. Argon is used in bulbs as inert gas. Kr and Xe are used in high efficiency lamps, head light of cars. Radon is radioactive formed by a-decay of Radium 226 88Ra Argon is most abundant (0.9%) noble gas in atmosphere.
The following questions are multiple choice questions. Choose the most appropriate answer.
1) What are the elements in group 18 (the far right) of the periodic table called?
a) Alkali metals
b) Alkaline earth metals
c) Halogens
d) Noble gases
2) Out of (i) \(XeO_3\) (ii) \(XeOF_4\) and (iii) \(XeF_6\) , the molecules having the same number of lone pairs on Xe are -
a) (i) and (ii) only
b) (i) and (iii) only
c) (ii) and (iii) only
d) (i) , (ii) and (iii)
3) Which one has linear shape?
a) \(XeF_2\)
b) \(XeF_4\)
c) \(XeF_6\)
d) \(XeO_3\)
4) Which of the outer electronic configuration represent Argon?
a) \(ns^2\)\(np^4\)
b) \(ns^2\)\(np^3\)
c) \(ns^2\)\(np^6\)
d) \(ns^1\)\(np^6\)
5) Which of the following statement is false?
a) Radon is obtained from the decay of radium
b) Helium is an inert gas
c) Xenon is the most reactive among the rare gases
d) The most abundant rare gas found in the atmosphere is helium
Molecular Nitrogen \(N_2\) comprises about 78% by volume of Earth’s atmosphere. It occurs as Sodium nitrate, \(NaNO_3\)(chile saltpeter) & Potassium nitrate, \(KNO_3\)(Indian altpeter) in earth’s crust. Since nitrate are very soluble in water so these are not wide spread in the earth’s crust. Nitrogen is also an important constituent of amino acids, protein & nucleic acids in plants & animals.
Nitrogen shows anomalous behavior from rest of the elements due to following reasons;
Smaller size, high ionization enthalpy, high electronegativity & absence of d-orbital. It has unique ability to form p∏-p∏ multiple bonds with itself & with small size atoms like C & O as they have small size & high electronegativity. Heavier elements of this group do not form p∏-p∏ bonds as their atomic orbitals are so large & diffuse that they can’t have effective overlapping.
Thus Nitrogen exists as diatomic molecules \(N_2\) with a triple bond. Consequently, its bond enthalpy (941.4 KJ \(mol^{-1}\)) is very high. P, As & Sb form only single bonds as P-P, As-As & Sb-Sb. Due to much bond enthalpy N is much less reactive than P.
Single N-N bond is weaker than single P-P bond due to high interelectronic repulsion of the non bonding electrons, owing to small bond length. As a result, the catenation tendency is weaker in nitrogen. Hence nitrogen exists as gas while phosphorus exists as solid.
Nitrogen can’t form d∏- d∏ bond due to absence of d- orbitals so it can’t expand its covalency beyond four as heavier members can.
The following questions are multiple choice questions. choose the most appropriate answer.
1) Among group 15 elements which exists as gas at room temperature
a) Arsenic
b) Bismuth
c) Nitrogen
d) Phosphorous
2) The stability of +5 oxidation state decreases and that of +3 state increases down the group in group 15 elements due to
a) inert pair effect
b) decrease in ionisation enthalpy
c) increase in size
d) shielding effect
3) Nitrogen is restricted to a maximum covalency of 4 because of
a) absence of d-orbitals
b) presence of d-orbitals
c) absence of s and p-orbitals
d) none of the above
4) Extra pure \(N_2\) can be obtained by heating
a) \(NH_3\) with CuO
b) \(NH_4NO_3\)
c) \(\left(NH_4\right)_2Cr_2O_7\)
d) \(Ba\left(N_3\right)_2\)
5) Catenation tendency is weaker in nitrogen, because of
a) single N–N bond is weaker
b) single N–N bond is stronger
c) ability to form pi bonds by N atoms
d) none of the above
Group 16 elements are called chalcogens i.e., ore forming elements (oxygen, sulphur, selenium etc.) because most of the ores are oxides and sulphides. Oxygen is gas where as other elements of group 16 are solids. Oxygen shows anomalous behaviour. Oxygen is diatomic where is sulphur exists as \(S_8\) which has crown shaped structure. It shows allotropy. Sulphur is present in onion and garlic that is why they have pungent smell. Sulphur is used for manufacture of sulphuric acid which is called ‘King of chemicals’, used in fertilizer, detergents, dyes and drugs.
The following questions are multiple choice questions. Choose the most appropriate answer.
1) Group 16 elements are also known as
a) Noble elements
b) Halogens
c) Pnictogens
d) Chalcogens
2) Acidic character of hydrides of group 16 elements is in the order
a) \(H_2\)O < \(H_2\)S < \(H_2\)Se < \(H_2\)Te
b) \(H_2\)S < \(H_2\)Se < \(H_2\)Te < \(H_2\)O
c) \(H_2\)O < \(H_2\)Se < \(H_2\)Te < \(H_2\)S
d) \(H_2\)O < \(H_2\)S < \(H_2\)Te < \(H_2\)Se
3) Hybridisation of S in \(SF_4\) and geometry of \(SF_4\) are respectively
a) \(sp^3\)d, trigonal pyramidal
b) \(sp^3\)d, see saw
c) \(sp^3\), tetrahedral
d) \(dsp^2\), square planner
4) Which is not an acidic oxide?
a) \(CO_2\)
b) \(SO_2\)
c) \(Na_2\)O
d) \(Cl_2\)\(O_7\)
5) Which is not correct about allotropes of sulphur
a) The stable form at room temperature is rhombic sulphur
b) Monoclinic sulphur is stable above 369 K and transforms into rhombic sulphur below it
c) At 369 K both the forms are stable
d) Monoclinic sulphur is soluble in \(CS_2\) while rhombic sulphur not
The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties. Relative lowering in vapour pressure is also an example of colligative properties. For an experiment, sugar solution is prepared for which lowering in vapour pressure was found to be 0.061 mm of Hg.(vapour pressure of water at \(20^\circ\)C is 17.5 mm of Hg).
Answer the following MCQs by choosing the most appropriate options:
(i) Relative lowering of vapour pressure for the given solution is-
(a) 0.00348
(b) 0.061
(c) 0.122
(d) 1.75
(ii) The vapour pressure (mm of Hg) of Solution will be
(a) 17.5
(b) 0.61
(c) 17.439
(d) 0.00348
(iii) Mole fraction of sugar in the solution is
(a) 0.00348
(b) 0.9965
(c) 0.061
(d) 1.75
(iv) If weight of sugar taken is 5 g in 108 g of water then molar mass of sugar will be
(a) 358
(b) 120
(c) 240
(d) 400
(v) The vapour pressure (mm of Hg) of water at 293 K when 25 g of glucose is dissolved in 450 g of water is
(a) 17.2
(b) 17.4
(c) 17.120
(d) 17.02
Read the passage given below and answer the following questions:
When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
The denaturation causes change in secondary ann tertiary structures but primary structures remain intact. Examples of denaturation of protein are coagulation of egg white on boiling, curdling of milk, formation of cheese when an acid is added to milk.
Choose the most appropriate answer:
i) Mark the wrong statement about denaturation of proteins.
a) The primary structure of the protein does not change.
b) Globular proteins are converted into fibrous proteins.
c) Fibrous proteins are converted into globular proteins.
d) The biological activity of the protein is destroyed.
ii) Which structure{s) of proteins remains(s) intact during denaturation process?
(a) Both secondary and tertiary structures
(b) Primary structure only
(c) Secondary structure only
(d) Tertiary structure only
iii) a-helix a n d β - pleated structures of proteins are classified as
(a) primary structure
(b) secondary structure
(c) tertiary structure
(d) quaternary structure.
(iv) Secondary structure of protein refers to
a) mainly denatured proteins and structure of prosthetic groups.
b) three-dimensional structure, especially the bend between. amino acid residues that are distant from each other in the polypeptide chain.
c) linear sequence of amino acid residues in the polypeptide chain.
d) regular folding patterns of continuous portions of the polypeptide chain.
Read the passage given below and answer the following questions:
The transition metals when exposed to oxygen at low and intermediate temperatures form thin, protective oxide films of up to some thousands of Angstroms in thickness. Transition metal oxides lie between the extremes of ionic and covalent binary compounds formed by elements from the left or right side of the periodic table. They range from metallic to semiconducting and deviate by both large and small degrees from stoichiometry. Since d electron bonding levels are involved, the cations exist in various valence states and hence give rise to a large number of oxides. The crystal structures are often classified by considering a cubic or hexagonal close-packed lattice of one set of ions with the other set of ions filling the octahedral or tetrahedral interstices. The actual oxide structures, however, generally show departures from such regular arrays due in part to distortions caused by packing of ions of different size and to ligand field effects. These distortions depend not only on the number of d-electrons but also on the valence and the position of the transition metal in a period or group.
In the following questions, a statement of assertion followed by a statement of reason is given.
Choose the correct answer out of the following choices on the basis of the above
passage.
A. Assertion and reason both are correct statements and reason is correct explanation for assertion.
B. Assertion and reason both are correct statements but reason is not correct explanation for assertion.
C. Assertion is correct statement but reason is wrong statement.
D. Assertion is wrong statement but reason is correct statement.
1) Assertion: Cations of transition elements occur in various valence states
Reason: Large number of oxides of transition elements are possible.
2) Assertion: Crystal structure of oxides of transition metals often show defects.
Reason: Ligand field effect cause distortions in crystal structures.
3) Assertion : Transition metals form protective oxide films.
Reason: Oxides of transition metals are always stoichiometric.
4) Assertion: CrO crystallises in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by chromium ions.
Reason: Transition metal oxide may be hexagonal close-packed lattice of oxide ions with metal ions filling the octahedral voids.
The solubility of gases increases with increase of pressure. William Henry made a systematic investigation of the solubility of a gas in a liquid. According to Henry’s law “the mass of a gas dissolved per unit volume of the solvent at constant temperature is directly proportional to the pressure of the gas in equilibrium with the solution”. Dalton during the same period also concluded independently that the solubility of a gas in a liquid solution depends upon the partial pressure of the gas. If we use the mole fraction of gas in the solution as a measure of its solubility, then Henry’s law can be modified as “the partial pressure of the gas in the vapour phase is directly proportional to the mole fraction of the gas in the solution”.
Answer the following MCQs by choosing the most appropriate options:
(i) Henry’s law constant for the solubility of methane in benzene at 298 K is 4.27 × \(10^5\) mm Hg. The solubility of methane in benzene at 298 K under 760 mm Hg is
(a) 4.27 × \(10^{-5}\)
(b) 1.78 × \(10^{-3}\)
(c) 4.27 × \(10^{-3}\)
(d) 1.78 × \(10^{-3}\)
(ii) The partial pressure of ethane over a saturated solution containing 6.56 × \(10^{-2}\) g of ethane is 1 bar. If the solution contains 5.00 × \(10^{-2}\) g of ethane then what will be the partial pressure (in bar) of the gas?
(a) 0.762
(b) 1.312
(c) 3.81
(d) 5.0
(iii) KH (K bar) values for Ar(g), \(CO_2\)(g), HCHO(g) and \(CH_4\)(g) are 40.39, 1.67, 1.83 × \(10^{-5}\) and 0.413 respectively. Arrange these gases in the order of their increasing solubility.
(a) HCHO < \(CH_4\) < \(CO_2\) < Ar
(b) HCHO < \(CO_2\) < \(CH_4\) < Ar
(c) Ar < \(CO_2\) < \(CH_4\) < HCHO
(d) Ar < \(CH_4\) < \(CO_2\) < HCHO
(iv) When a gas is bubbled through water at 298 K, a very dilute solution of the gas is obtained. Henry’s law constant for the gas at 298 K is 150 K bar. If the gas exerts a partial pressure of 2 bar, the number of millimoles of the gas dissolved in 1 L of water is
(a) 0.55
(b) 0.87
(c) 0.37
(d) 0.66
