The crystal showing defect is:
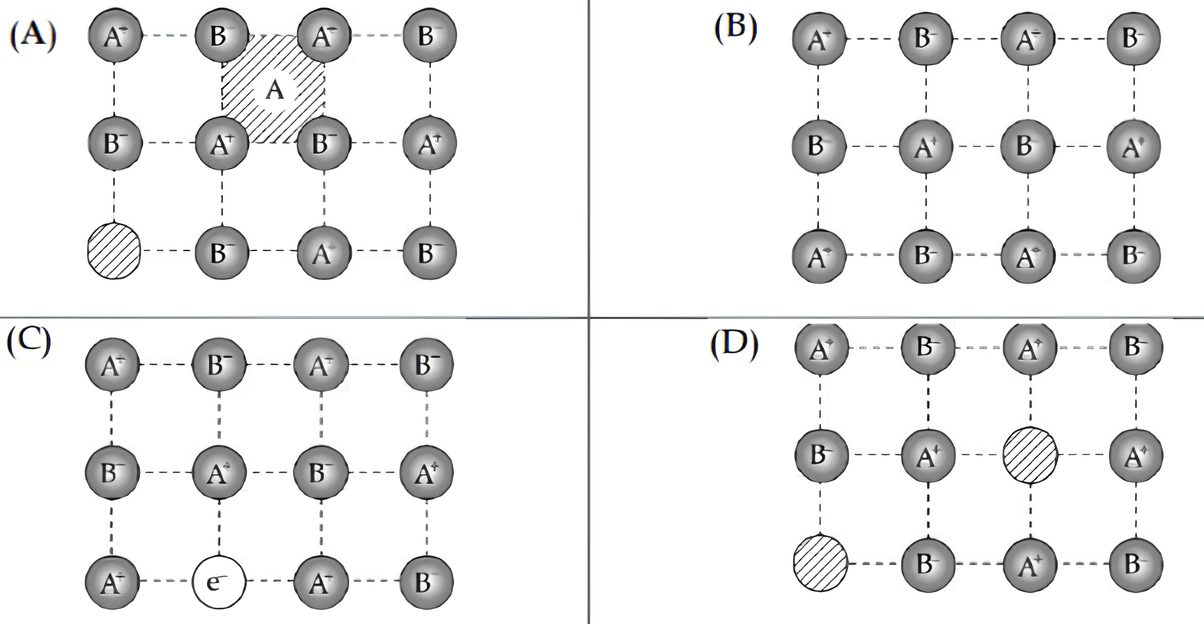

(A) 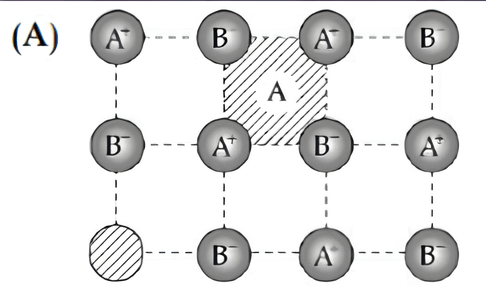

The total number of tetrahedral voids in the face centred unit cell is _____.
(A) 6
(B) 8
(C) 10
(D) 12
The lattice site in a pure crystal cannot be occupied by __________.
(A) Molecule
(B) Ion
(C) Electron
(D) Atom
What is the coordination number in a square close packed structure in two dimensions?
(A) 2
(B) 3
(C) 4
(D) 6
Graphite cannot be classified as __________.
(A) Conducting solid
(B) Network solid
(C) Covalent solid
(D) Ionic solid
Which of the following is an amorphous solid?
(A) Graphite (G)
(B) Quartz glass (SiO2)
(C) Chrome alum
(D) Silicon carbide (SiC)
Which stoichiometric defect does not change the density of the crystal?
(A) Frenkel defect
(B) Schottky defect
(C) Interstitial defect
(D) F-centres
What would be the reactant and reagent used to obtain 2, 4-dimenthyl pentan-3-ol?
(a) Propanal and propyl magnesium bromide.
(b) 3-methylbutanal and 2-methyl magnesium iodide.
(c) 2-dimethylpropanone and methyl magnesium iodide.
(d) 2-methylpropanal and isopropyl magnesium iodide.
Which of the following statements is not true about the hexagonal close packing?
(A) The coordination number is 12.
(B) It has 74% packing efficiency.
(C) Tetrahedral voids of the second layer are covered by the spheres of the third layer.
(D) In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
Interstitial compounds are formed when small atoms are dropped under the curved lattice of metals. Which of the following is not the characteristics property of interstitial compounds?
(A) They have high melting points in to pure metals
(B) They are very hard
(C) They retain metallic Conductivity
(D) They are chemically very reactive
What is the coordination number in a square close packed structure in two dimensions?
(A) 2
(B) 3
(C) 4
(D) 6
Which statement is incorrect about peptide bond?
(a) C-N bond length in proteins is longer than usual bond length of C-N bond.
(b) Spectroscopic analysis shows planar structure of -CO-NH- group
(c) C-N bond length in proteins is smaller than usual bond length of C-N bond
(d) None of the above
RNA and DNA are chiral molecules. Their chirality is due to :
(a) Chiral bases
(b) Chiral phosphate ester units
(c) D-sugar component
(d) L-sugar component.
When Benzene diazonium chloride is treated with cuprous chloride in HCl, Chlorobenzene is formed, This reaction is known as –
a) Etard Reaction
b) Perkin’s Reaction
c) Gattermann’s Reaction
d) Sand Meyer’s Reaction
