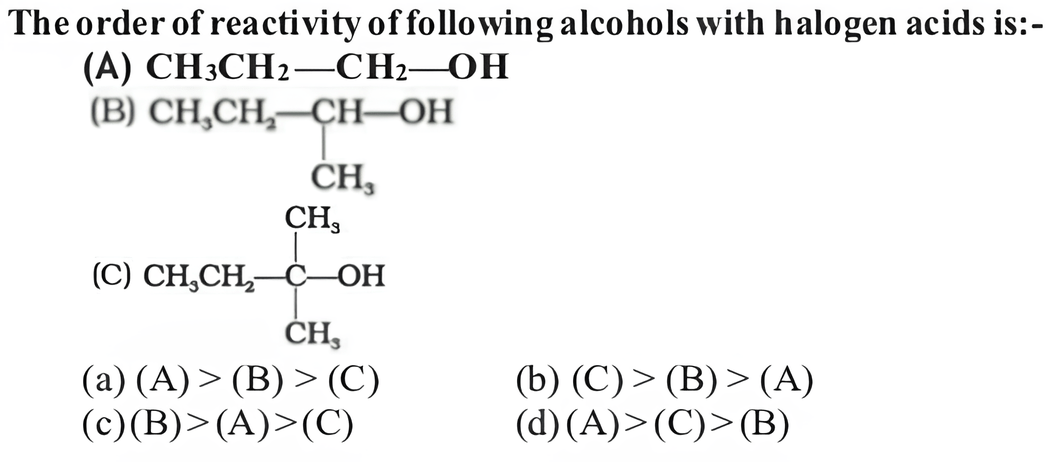
(b) (C) > (B) > (A)
Write the structure of an isomer of compound C₄H₉Br which is most reactive towards reaction.
Which of the following reagents cannot, be used to oxidise primary alcohols to aldehydes?
(a) \(CrO_3\) in anhydrous medium.
(b) \(KMnO_4\) in acidic medium.
(c) Pyridinium chlorochromate.
(d) Heat in the presence of Cu at 573 K.
Which of the following is not true about the ionic solids?
(A) Bigger ions form the close packed structure.
(B) Smaller ions occupy either the tetrahedral or the octahedral voids depending upon their size.
(C) Occupation of all the voids is not necessary.
(D) The fraction of octahedral or tetrahedral voids occupied depends upon the radii of the ions occupying the voids.
Which of the following statements is not true about the hexagonal close packing?
(A) The coordination number is 12.
(B) It has 74% packing efficiency.
(C) Tetrahedral voids of the second layer are covered by the spheres of the third layer.
(D) In this arrangement spheres of the fourth layer are exactly aligned with those of the first layer.
Which of the following is an amorphous solid?
(A) Graphite (G)
(B) Quartz glass (SiO2)
(C) Chrome alum
(D) Silicon carbide (SiC)
When mercuric iodide is added to the aqueous solution of potassium iodide, the:
(a) Freezing point is raised
(b) Freezing point does not change
(c) Freezing point is lowered
(d) Boiling point does not change
