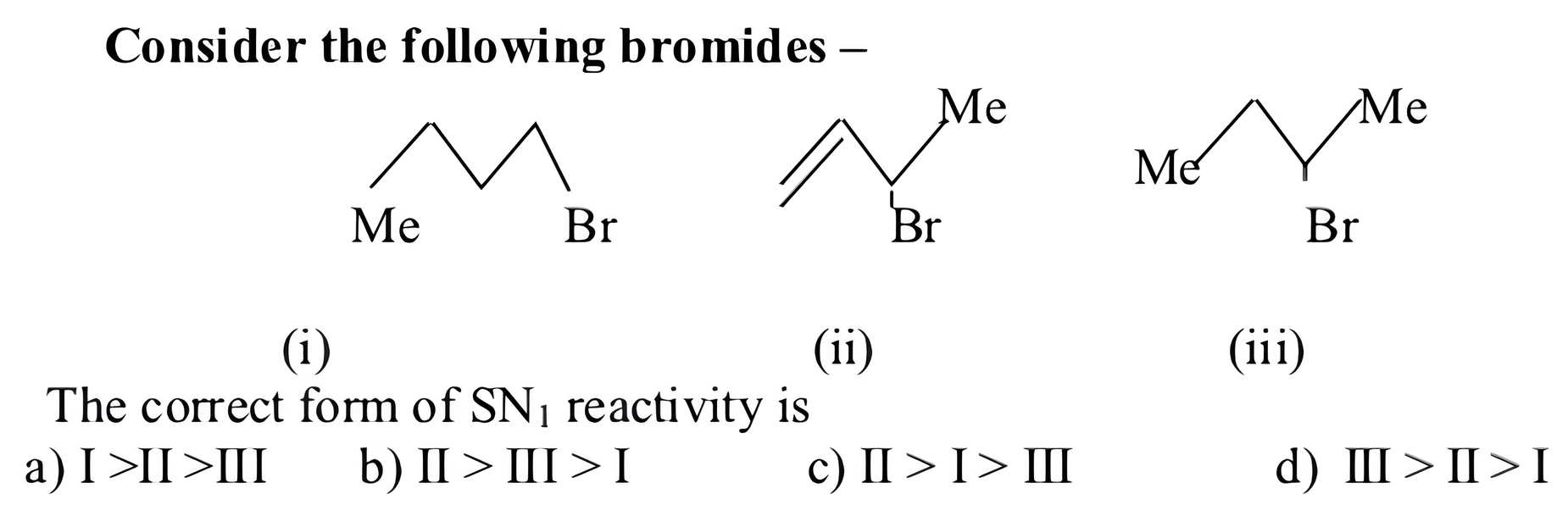
b)II > III > I
Write the structure of an isomer of compound C₄H₉Br which is most reactive towards reaction.
\(SO_2\) is ----------and \(TeO_2\) is -------- agent
a) reducing, an oxidising
b) an oxidising, reducing
c) reducing, reducing
d) an oxidising, an oxidising
The ionisation enthalpy of the group 15 elements is much greater than that of group 14 elements in the corresponding periods. Which is the most suitable reason?
a) more effective nuclear charge
b) presence of stable half-filled electronic configuration
c) smaller size
d) high electronegativity
RNA and DNA are chiral molecules. Their chirality is due to :
(a) Chiral bases
(b) Chiral phosphate ester units
(c) D-sugar component
(d) L-sugar component.
Chlorine water on standing loses its yellow colour due to the formation of
a) Cl and HOCl.
b) HCl and HOCl.
c) HOCl and \(HOCl_2\).
d) HCl and \(HOCl_2\).
Which of the following statement is false?
a) Radon is obtained from the decay of radium
b) Helium is an inert gas
c) Xenon is the most reactive among the rare gases
d) The most abundant rare gas found in the atmosphere is helium
The molal elevation constant depends upon
(a) nature of solute.
(b) nature of the solvent.
(c) vapour pressure of the solution.
(d) enthalpy change.
