Write the structure of an isomer of compound C₄H₉Br which is most reactive towards reaction.
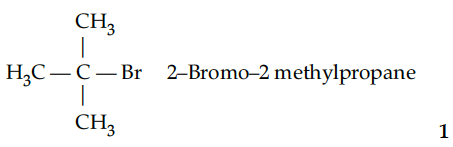
How would you account for the following ? Many of the transition elements are known to form interstitial compounds.
Explain boiling point elevation constant for a solvent or ebullioscopic constant.
Define the following terms :
(i) Isotonic solutions
(ii) Van’t Hoff factor
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number.
Explain the following observation :
Most of the transition metal ions exhibit characteristic colour in aqueous solution.
