Metallic radii of some transition elements are given below. Which of these elements will have highest density?
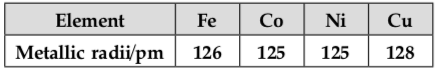
(a) Fe
(b) Ni
(c) Co
(d) Cu

(a) Fe
(b) Ni
(c) Co
(d) Cu
(d) Cu
Explanation: In periodic table when moving from left to right along period, its metallic radius decreases and mass increases. Decrease in metallic radius coupled with increase in atomic mass which results in the increase in density of metal. Therefore, among above four options, copper belongs to right side of periodic table in transition metal and it has the highest density.
Explanation: In periodic table when moving from left to right along period, its metallic radius decreases and mass increases. Decrease in metallic radius coupled with increase in atomic mass which results in the increase in density of metal. Therefore, among above four options, copper belongs to right side of periodic table in transition metal and it has the highest density.
Electronic configuration of a transition element X in +3 oxidation state is [Ar]3d⁵. What is its atomic number?
(a) 25
(b) 26
(c) 27
(d) 24
What are the transition elements ? Write two characteristics of the transition elements.
Write the formula of an oxo-anion of Manganese (Mn) in which it shows the oxidation state equal to its group number.
Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number.
How would you account for the following ? Many of the transition elements are known to form interstitial compounds.
Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with (i) an iodide (ii) H₂S.
Assign the reason for the following :
Copper (I) ion is not known in aqueous solution.
Graphite cannot be classified as __________.
(A) Conducting solid
(B) Network solid
(C) Covalent solid
(D) Ionic solid
Low concentration of oxygen in the blood and tissues of people living at high altitude is due to-
(a) low temperature
(b) low atmospheric pressure
(c) high atmospheric pressure
(d) both low temperature and high atmospheric pressure
Hypochlorous acid and perchloric acid are, respectively:
a) HOCl and \(HClO_4\).
b) HOCl and \(HClO_3\).
c) \(HClO_2\) and \(HClO_3\).
d) \(HClO_2\) and \(HClO_4\).
The letter ‘D’ in carbohydrates signifies-
(a) dextrorotatory
(b) configuration
(c) diamagnetic nature
(d) mode of synthesis
In the periodic table of the elements, the phrase “middle row anomaly” refers to
a) Middle elements of periodic table are transition metals.
b) Middle element of each group is unstable.
c) the relative instability of bromine oxides compared to the other halogen oxides.
d) The higher oxides of halogens tend to be more stable than the lower ones.
The reason for double helical structure of DNA is operation of-
(a) electrostatic attractions
(b) dipole-dipole interaction
(c) van der Waal’s forces
(d) hydrogen bonding
Interstitial compounds are formed when small atoms are dropped under the curved lattice of metals. Which of the following is not the characteristics property of interstitial compounds?
(A) They have high melting points in to pure metals
(B) They are very hard
(C) They retain metallic Conductivity
(D) They are chemically very reactive
